Abstract
Introduction. Despite introduction of bortezomib-based regimens in the treatment of light-chain (AL) amyloidosis, early death remains a frequent complication particularly for patients (pts) with advanced cardiac involvement such as Mayo stage 3B. Daratumumab, an IgGκ monoclonal anti-CD38 antibody, has proven effective and tolerable in pts with AL amyloidosis, either alone or in combination with bortezomib-cyclophosphamide-dexamethasone (D-VCd). However, patients with Mayo stage 3B disease had been excluded from enrollment in the registration trial of D-VCd. This study evaluates the efficacy and safety of daratumumab monotherapy in newly diagnosed (ND) stage 3B AL amyloidosis pts.
Methods. The ongoing EMN22 phase 2, open-label, multinational study (NCT04131309) aims to enroll 40 ND pts with stage 3B AL amyloidosis. Eligible adult pts have N-terminal pro-brain natriuretic peptide (NT-proBNP) ≥8500 pg/mL and high-sensitivity troponin T (hsTnT) >54 pg/mL. Daratumumab monotherapy, was initially administered intravenously (16 mg/mL), and since February 2020 subcutaneously as a fixed dose of 1800 mg weekly during cycles (C)1 and 2, every 2 weeks for C3-6, and every 4 weeks thereafter. Pts not achieving a hematological very good partial response (VGPR) or better by C4 can receive additional weekly bortezomib and low dose dexamethasone (Vd). Treatment continues for up to 2 years from initiation or until disease progression or initiation of a new therapy. The primary endpoint is OS rate at 6 months. This analysis includes pts who initiated study treatment ≥6 months before data cut-off (31 May 2022); median (95% confidence interval [CI]) OS was obtained by Kaplan-Meier analysis.
Results. Twenty-nine pts were included in this analysis, of whom 6 (20.7%) remain on treatment by data cut-off and 23 (79.3%) had discontinued. Pts were mostly males (18, 62.1%), median age was 68 years (range 45-84). At screening, 20 (69%) pts had Eastern Cooperative Oncology Group performance status ≥2, and 11 (37.9%) and 18 (62.1%) pts had New York Heart Association class II and IIIA symptoms, respectively. Median NT-proBNP was 15,468 pg/mL (range 8,816-72,522), median hsTnT was 138.9 pg/mL (range 59.8-692), and the difference of involved to uninvolved free light chains was 452 (range 449-2823) mg/L. Besides the heart, 25 (86.2%) pts had ≥1 organs involved, most frequently the kidneys (14/25 pts, 56.0%) and the peripheral nerves (12/25 pts, 48.0%).
At a median follow-up of 9.4 months (range <0.1, 31.9), the median number of daratumumab administrations was 18 (range 1-36) with median treatment duration of 7.3 months (range <0.1-24.4). Seven (24.1%) pts received additional Vd treatment. The median OS was 9.4 months (95% CI 3.4, not reached) and the 3- and 6-month OS rates were 72.4% (95% CI 52.3, 85.1) and 62.1% (95% CI 42.1, 76.9), respectively.
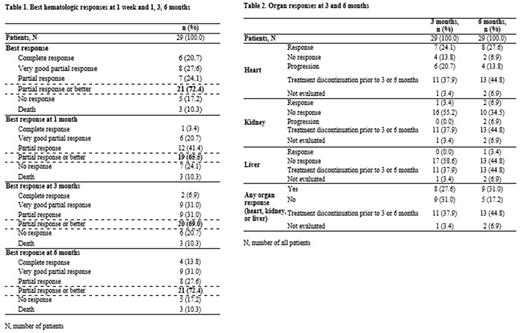
Best hematologic response (partial response [PR] or better) was achieved by 21 (72.4%) pts (complete response: 6 [20.7%]; VGPR: 8 [27.6%]; PR: 7 [24.1%]), all pts achieved a response within 6 months and 14 (48.3%) pts achieving a response within 1 week of treatment initiation (Table 1). Any organ response at 3 or 6 months was achieved by 8 (27.6%) and 9 (31.0%) pts, respectively; cardiac response rates at 3 and at 6 months were observed in 7 (24.1%) and 8 (27.6%) patients respectively (Table 2).
Twenty-eight (96.6%) pts had ≥1 non-serious adverse event (NSAE). Grade (Gr) 3/4 NSAEs occurred in 18 (62.1%) pts, commonly dyspnea and peripheral edema (4 [13.8%] pts each); 5 (17.2%) pts experienced treatment-related Gr3/4 NSAEs including Gr3 neutropenia related to daratumumab and Gr3 Troponin increase related to bortezomib. Twenty-two (75.9%) pts had ≥1 serious adverse event (SAE), including 16 (55.2%) pts with cardiac disorders and 12 (41.4%) pts with a fatal SAE. Treatment-related SAEs occurred in 4 (13.8%) pts and daratumumab-related SAEs in 2 (6.9%) pts (pneumonia Gr3 and sepsis Gr5).
Conclusions. In this high risk (stage 3B) patient population, daratumumab monotherapy demonstrates clinically meaningful hematologic and cardiac responses with no new safety signals. Compared to historic controls, early mortality is reduced and the median OS of stage 3B pts has been improved suggesting that novel treatments can help change the course of AL Amyloidosis even in pts at advanced stages of the disease. The study completed accrual and updated results will be presented in the meeting.
Disclosures
Kastritis:Takeda: Honoraria; Pfizer: Honoraria, Research Funding; GSK: Honoraria; Genesis Pharma: Honoraria; Janssen: Honoraria, Research Funding; Amgen: Honoraria, Research Funding. Minnema:Medscape: Speakers Bureau; Janssen-Cilag: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Speakers Bureau. Dimopoulos:Amgen: Honoraria; TAKEDA: Honoraria; BeiGene: Honoraria; Jannsen: Honoraria; BMS: Honoraria. Belhadj:BMS, JANSSEN, SANOFI, AMGEN, ABBVIE: Consultancy, Honoraria, Other: Travel for ASH, ASCO and EHA Annual Meeting. Varghese:Health Data Specialists: Current Employment. Manousou:Health Data Specialists: Current Employment. Sonneveld:Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees. Palladini:Sebia: Honoraria; Argobio: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gate Bioscience: Research Funding; The Binding Site: Honoraria, Research Funding; Protego: Honoraria, Membership on an entity's Board of Directors or advisory committees; Alexion: Honoraria, Membership on an entity's Board of Directors or advisory committees; Prothena: Honoraria; Pfizer: Honoraria; Siemens: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal